Services
The time & cost savings of an integrated approach to developing products, not constrained by the boundaries of different departments, has been realized in smart cross-functional organizations. STC Biologics, Inc. is founded on such boundary-less merge of disciplines, as the knowledge of Biology, Process Development, Analytics, Formulation, & Regulatory Sciences is in one team body. Our pride in technical rigor, perseverance, critical thinking, and multidisciplinary collaborative work ethics bring agile resolution to technical hurdles and nimble development of products.
CMC Consulting
As passionate developers of biotechnology products, we take it upon ourselves to become the experts on your product. We help our clients with phase-appropriate CMC strategy, while clearly outlining the risks and risk mitigation plans. Our management team has two decades of experience in the development of biologics, having written over 20 comparability protocols, numerous specification justifications, two dozen INDs, and 8 BLAs, with experience in the life cycle management of 5 approved biologics.We are uniquely positioned to guide our clients to develop an integrated CMC plan tied to preclinical and clinical development with a constant eye on regulatory expectations. We can help you through any aspect of CMC development, from technical development to CMO management, from design of studies to proposal reviews, from data reduction to writing reports and summary presentations.We customize our CMC consulting to your needs in the below areas:- Process development and process characterization
- Analytical development, qualification, and validation
- Formulation development and drug product process development
- Forced degradation studies
- Comparability strategy, execution, and data analysis
- Specifications development, process trending, and statistical analysis
- Product and impurity characterization
- Stability program design and shelf life estimation
- CMC regulatory (see Regulatory Sciences Services)
Cell Line Development
The selection of an expression system is determined by its ability to deliver high productivity with acceptable product quality. More than 70% of recombinant protein products are produced in CHO cells, typically from DUXB11, DG44, and CHOK1 lineages. NS0 and Sp2/0 murine myeloma cell lines, as well as PER.C6® human cells. Cell line development starts with construction of an expression vector carrying the cDNA encoding the recombinant protein. After vector transfection, host cells are subjected to selection pressure using selectable markers. To generate the cell line with adequate productivity and stability, hundreds to thousands of clones may have to be screened, first using only titer as selection criterion, and subsequently screening for cell growth and other cell characteristics, such as cell number, specific productivity, volumetric productivity, and clone stability, to enable selection of the top 4–6 clones for further evaluation in bioreactors, and ultimately selecting a final production clone with a backup clone.
For biosimilar development, many companies screen up to 1,000 clones to identify the clones with the desired quality attributes; however, here at STC Biologics, the number of clones screened is drastically reduced since we have devised process methods to decouple the productivity characteristics from the critical quality attributes, such as glycosylation. This platform allows us to conduct process development activities on only the two best producers to develop a highly biosimilar product.
In-house capabilities:
- CHO, NS0, Sp2/0 cell line development, including vector construction, transfection, titer and cell characteristics assessment
- Stable cell clone selection
- Research cell bank development
- Oversight of GMP cell bank generation and characterization
- Development of cell lines expressing biosimilar products
Process Development
Before initiating process development activities, target product and process profile is defined based on the available knowledge of the product class; for non-MAb novel products, the limited initial knowledge is supplemented by characterization and structure-activity studies conducted in parallel to process development. We have demonstrated that monitoring product quality and process robustness throughout process development is essential for producing a high quality product.
Robust implementation of cell culture technology requires optimization of a number of variables, including (1) cell lines capable of producing the product at high yields; (2) reproducible and scalable upstream and downstream process that meets product quality needs; (3) appropriate culture monitoring methods that ensure batch to batch reproducibility; (4) process understanding to allow scale up; and (5) appropriate in-process analytical methods to track product quality.
At STC Biologics, in addition to cell growth and productivity assays, we apply a panel of in-process assays that include intact mass, glycan map (if the protein is glycosylated), ion exchange HPLC, and RP-HPLC or HIC (depending on the product); for downstream process development, SDS-PAGE, HCP ELISA, size exclusion HPLC, and other assays (depending on the product) are added to monitor the removal of impurities. Bioassays are used to assess the potency of the product (e.g. enzyme activity for enzyme products).
Upstream process development starts with media and feed optimization in shake flasks. Only fully chemically defined media consisting of amino acids, vitamins, trace elements, inorganic salts, lipids, and recombinant insulin or insulin-like growth factors are used. Growth conditions are optimized without compromising product quality by single-component titration, spent medium analysis, and medium blending. Various fed batch-feeding schemes are examined using a DOE approach. STC’s proprietary additives are used (if necessary) to modulate specific product attributes, such as glycosylation and charge variants.
A capture step is developed at this stage to enable analysis of product quality. Optimization of feed composition and feeding strategy requires consideration of nutrient consumption, by-product accumulation, and the balance between promoting growth versus volumetric productivity. Subsequently, the cell culture process is scaled up to 1-3 L in disposable bioreactors. At this stage, physical parameters (i.e. temperature, gas flow rate, and agitation speed) and chemical parameters i(i.e. dissolved oxygen and carbon dioxide, pH, osmolality, redox potential, and metabolite levels of substrate, amino acids, and waste by-products) are optomized. Biological parameters used for determining the physiological state of the culture include viability and viable cell concentration. A temperature shift is often explored from 37°C to 30–35°C starting at 48-96 hours post inoculation to retain cells in G1 phase longer, delaying the onset of apoptosis. Once the process is developed it can be scaled up to 50 L at STC to produce non-GMP material. Our set up involves Sartorius DCU with disposable 3L Mobius bioreactors, as well as 5L, 15L and 50L from New Brunswick/Eppendorf. With a productivity between 2-5 g/L, a 50 L bioreactor run can yield up to 250 g of protein, which is often sufficient to support pre-clinical and toxicology studies in animals.
Downstream process development is conducted in parallel by generating feeds at 1-3L scale, subjecting it first to depth filtration followed by a capture step.
A typical purification process for MAbs includes viral inactivation, one to two chromatographic steps designed to remove certain process and product impurities, viral removal filtration, and UF/DF to reach the target protein concentration and formulation composition. Cation and anion exchange chromatography are frequently utilized to obtain a highly purified product. Other chromatography resins such as HIC, CHT, MEP, or HEA can also be used to remove product and process specific impurities.
With extensive experience in pharmaceutical development and global commercialization of several biological products, STC Biologics can support your drug product development needs.
- Development of innovative, economic, and scalable processes to yield highly pure products
- Biosimilar and novel product process development
- Cell culture process development in 1L to 50 L single use bioreactors, including media and feed optimization
- Capture step and downstream purification process development (affinity chromatography, ion exchange and hydrophobic interaction chromatography, multi-mode and lectin chromatography), membrane separations
- Viral inactivation, viral filtration
- UF/DF (tangential flow filtration) from 10 ml to multi-liter scale
- Authoring the relevant 3.2.S section
Scale Down Process Characterization
Over the past few years STC Biologics has worked with a number of products that exhibited undesired toxicity or stability issues after they were brought into the clinic. These problems occurred because of the use of template processes and methods without sufficient understanding of the process and the product. Although some programs were able to fix the product/process issues, on average, they lost 6-12 months and spent between $2-10M dollars in clinical and manufacturing costs. At STC Biologics, we believe that early, abbreviated process and product characterization before pilot scale production can reduce the development risk and cost by defining the effects of process variables on the quality of the product. To characterize the process we will analyze the impact of different batches of media, temperature, DO and pH, feed amount and schedule on growth, titer and quality of the product. Understanding these parameters will ensure successful scale up and transfer between manufacturing facilities.
Analytical Development & Qualification
Based on the detailed characterization data in combination with our expertise in regulatory requirements for product control, we strategize the selection and development of release and stability assays. To examine the stability indicating nature of assays, we employ forced degradation kinetic samples selected to generate ~10-40% degradation, avoiding extensive structural change. We continue to select assays that are both sensitive to degradation and that show multiple degradation species. From the use of platform MAb analytical methods to developing assays for complex proteins and in-process testing, we can support your analytical development needs in all project phases.
In-house capabilities:
- HPLC assays
- Electrophoretic assays
- Immunoassays (ELISA), including antigen or receptor binding
- Cell based bioassays and statistical analysis
- Written Test Methods with detailed procedures and system suitability criteria to be followed every time
- Assay qualification per protocol and target criteria
- Assay development for preclinical studies
- Authoring relevant regulatory sections
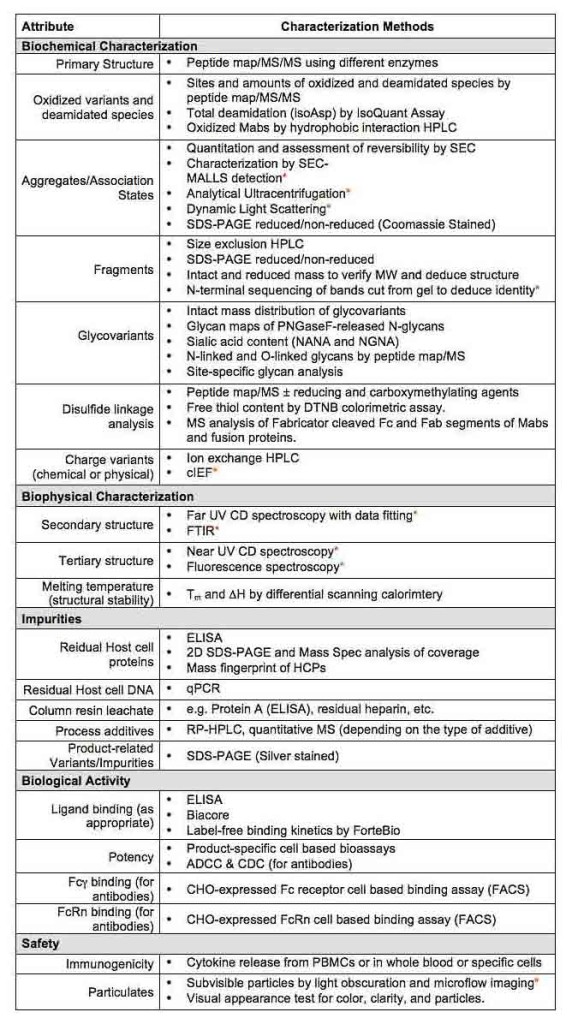
Product & Impurity Characterization / Comparability
At STC Biologics we have the capability to comprehensively characterize the structure, glycosylation, impurities, mechanisms of degradation, and biological activities of your biologics product (see our brochure on Integrating Bioassays into CMC Development). We apply orthogonal, specific, and sensitive analytical tools, including mass spectrometry (MS/MS on Q-ExactivePlus), HPLC, electrophoresis, ELISA, spectroscopy, and cell-based bioassays. Having established biosimilar product development characterization rigor, we analyze these data to generate a cohesive picture of the product and its unique features.
In-house capabilities:
- Peptide map UV and MS detection, bottom up analysis
- Intact mass, top down analysis
- Glycan analysis: Normal phase HPLC of 2-A labeled released glycans, intact mass of glycosylated and deglycosylated protein, MS analysis of released glycans
- Degradation product isolation and characterization
- Disulfide linkage analysis
- Size distribution analysis and characterization of aggregates
- Characterization of charge isoforms
- Authoring 3.2.S.1 and 3.2.S.2
- Developing comparability strategy per ICH Q5E
- Conducting comparability studies and statistical analysis of residual uncertainty
Mass Spectrometry Services
With our high resolution Q-Exactive Orbitrap mass spectrometer, we are able to conduct intact mass analysis of a 150 kDa antibody with 2-3 Da mass accuracy, differentiate 5-10% deamidation (+1 Da) in a 50 kDa protein by intact mass, and do semi-quantitative glycovariant distribution analysis.
We apply MS/MS feature to determine protein sequences and characterize degradation products and impurities in biological products. Target ID and HCP analysis is facilitated by peptide map/MS/MS combined with proteomics search tools.
Bioanalytical Assays (PK & Immunogenicity)
STC Biologics’ biological expertise combined with the regulatory knowledge has enabled us to establish robust bioanalytical services to measure concentration of your product in serum and blood. For each product we start with assay development to produce accurate and sensitive method of detection. We conduct MRD studies to understand the effect of matrix on accuracy of test article detection. To enable biologics development program, we can help your team establish PK and immunogenicity assays per regulatory guidance.
STC Biologics is in the process of establishing GLP testing capabilities to validate bioanalytical assays and conduct analysis on clinical and toxicology samples.
In-house capabilities:
- PK assay development and testing
- Immunogenicity assay development and testing
- Anti-drug antibody assay development and testing
Bioassays
Examination of the biological activity is of uttermost importance during all phases of drug development; for example, to identify a development candidate with a desired biological activity and to make decisions around process conditions that meet the desired product profile.
With broad knowledge of biology and experience in bioassay development, STC Biologics specializes in the development of custom biological assays specifically designed to examine your product’s mechanism of action. During assay development, critical assay parameters are identified and optimized for minimal variability. Each assay is qualified for specific sample matrix. Dose response curves are fitted for statistical best fit. When appropriate, relative potency is established in order to apply a parallel line analysis. System suitability criteria are established and selected assay parameters are statistically tracked to monitor assay performance. The stability indicating nature of the assay is assessed using forced degradation samples. Potency assays are qualified in early development and are later validated for their specificity, linearity, precision, robustness, and stability indicating nature.
In-house capabilities:
- Antigen- or receptor- binding by ELISA or cell based assays using FACS
- Panel of all Fc receptors for antibody products
- Cellular responses: growth factor-induced proliferation, growth arrest, apoptosis, cytokine release, growth factor-induced angiogenesis, including ADCC, CDC antibody products
- Signal transduction: examination of phosphorylation state of the receptor or its downstream signaling components, receptor down-regulation
- Gene transcription reporter assays

Formulation Development
With a successful development of formulations for 5 marketed products and dozens of clinical stage development molecules, STC Biologics offers an integrated approach to biomolecule’s structural and biochemical stabilization by characterization of degradation pathways and link to the history of the molecule’s production and handling. Such an approach facilitates optimization of the manufacturing process to produce a stable high quality product.
We begin formulation efforts with collecting information on the characteristics of the product, including primary sequence, pI, secondary and tertiary structures, potential sequence motifs susceptible to deamidation, oxidation and fragmentation, free thiols, etc. We have a systematic approach to designing forced degradation studies that would allow efficient development of stability indicating assays and highlight the types of degradation to stabilize against.
In-house capabilities:
- Preformulation studies, including biochemical and biophysical characterization. For instrumentation that is not available in-house, we have partnered with laboratories with such capability; nevertheless we have the expertise to interpret the results and apply them for development of formulations
- Formulation development, liquid or lyophilized, nanoparticles, liposomes
- Stability studies, long term and accelerated
- Forced degradation studies.
- Characterization of degradation pathways
- Formulation robustness studies
Release Testing & Stability Programs
STC Biologics is committed to high quality and well-documented processes, in line with GMP principles for product testing.
We plan to offer GMP release testing and stability program for biotherapeutics by Q1 2016. The Quality Manual has already been written, and work towards the necessary Quality Systems and laboratory requirements is underway.
STC Biologics can provide testing of the toxicology or preclinical batches, and set up stability studies under frozen, 2-8C, and accelerated conditions in support of those programs and in preparation for gaining experience with product’s stability profile.
In-house capabilities:
- Drug substance and drug product stability testing
- Real time, accelerated, and stress conditions
- HPLC, electrophoresis, appearance, particulates, bioassays
Technology Transfer
STC Biologics documents process and analytical development efforts in formats that are suitable for knowledge transfer to a GMP production site or testing laboratory. For transfer of analytical methods, qualified test methods containing system suitability criteria, and method qualification reports containing details of method understanding will be provided to the CRO.
STC has successfully transferred several drug substance or drug product processes to sites in the US and the EU, and is familiar with the logistics and challenges faced to achieve such successes. Our staff will be available for scientist-to-scientist discussions to ensure complete knowledge transfer.
In-house capabilities:
- Drug substance process transfer, including process development reports
- Drug product process transfer, including process development reports
- Analytical transfer, including signed off Test Methods and assay qualification reports
- Review of transfer protocols, process data trend analysis, troubleshooting
GMP production
STC has established GMP production capability since Q4 2019, now completing full CMC development services from cell line development to released drug substance. STC has 3 GMP clean rooms:
1) Suite 1 : 330 sq foot Master Cell Banking and Small Production Space 3.5- 100L
2) Suite 2 : 4000 sq ft GMP Production Facility up to 500L- 2x1000L
3) Suite 3. 200 sq ft GMP buffer and formulation prep area
Additional 11,000 sq ft of space in the same building has been procured as warehousing space as well as future gene therapy production area.
- Mammalian only
- Completely new construction (electrical/ HVAC/plumbing)
- Walkable ceiling
- WFI purchased (no drains, no sinks)
- EU room classification
- Controlled access
- Controlled personnel and material flow
- Already established Quality Systems